Intercalation of Solid C60 with Iodine
Qing Zhu, John E. Fischer, Krzysztof Kniaz, Andrew R. McGhie, Otto Zhou
Laboratory for Research on the Structure of Matter University of Pennsylvania, Philadelphia PA 19104-6272
David E. Cox
Department of Physics, Brookhaven National Laboratory Upton, NY 11973
Image
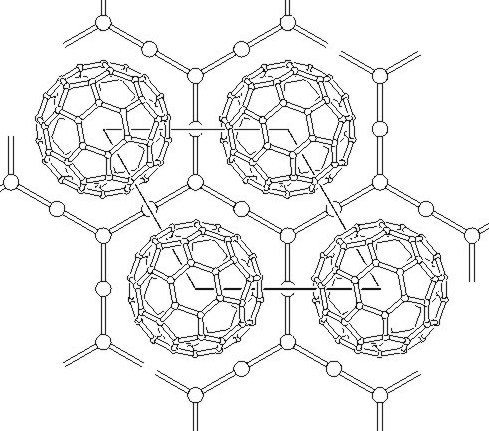
A view (along the 00l direction) of an idealized C60I4 shows nice primitive-hexagonal, layered (A-B-A) structure of the compound. Best refinement was obtained for a disordered model, in which iodines are located off the high symmetry positions with some of sites vacant giving the real stochiometry of C60I3.64.
Text
Metallic and superconducting donor-type intercalation compounds of C60 are now well-established. These involve electron transfer from a sublattice of alkali metal dopants to a sublattice of fullerene molecules. Three stoichiometric phases have been identified, and a binary phase diagram describing composition regions of two-phase coexistence has been proposed. On the other hand, oxidative intercalation by electron acceptors has not been reported to date. In a recent note, Ohno et al. presented photoemission evidence that only minor amounts of iodine are taken up by solid C60, resulting in a non-metallic product which is not a definite compound. Using different reaction conditions, we readily obtained a highly crystalline phase of ideal stoichiometry C60I4 whose alternating guest-host layer structure closely resembles that of classic intercalation compounds. The 300 K resistivity exceeds 10^9 [Omega/] and superconductivity is not observed above 4 K, despite the fact that the inter-fullerene separation lies in the range of the superconducting M3C60 phases (M = K, Rb, Cs). C60I4 is apparently the first example of a fullerite intercalation compound with no electron transfer between C60 and the intercalate.
Discussion of the possibility of a superconducting phase in iodine-doped C60 invoked the idea of acceptor-type fullerene ``salts'', resulting for example from oxidative intercalation by halogens. This would appear to be ruled out a priori by the large C60 molecule ionization potential and the absence of reversible oxidation steps in solution electrochemistry, although the electrostatic penalty per molecule could be partially offset by the Madelung energy of a resulting ionic lattice. On the other hand, there are many examples of synthetic metals obtained by halogen doping into other carbon-based hosts. Iodine is the dopant of choice for testing the effect of polymer morphology on the conductivity of polyacetylene compounds; bromine and the inter-halogens IBr and ICl intercalate readily into graphite at 300 K either from the vapor or by immersing graphite in halogen-CCl4 solutions. These are generally p-type metals, and counter-ions such as $I3^- and I_5^- have been identified.
Vapor phase reactions of iodine with pure C60 were carried out at 250 deg C for several days in evacuated pyrex tubes, using starting mixtures I:C60 1, 2, 3 and 12. The C60 was maintained a few degrees hotter than the iodine to prevent direct contact between C60 and condensed I2 and to discourage excess I2 from crystallizing on the sample upon cooldown. The saturation I2 pressure at 250 deg C is 3-4 atmospheres. X-ray powder diffraction profiles measured at beamline X10B of the National Synchrotron Light Source at Brookhaven National Laboratory (wavelength 0.9470 Angstr) revealed a single, highly crystalline new pattern in all four samples. Substantial amounts of C60 (face-centered cubic, a = 14.17 Angstr.) were also detected in the samples prepared from C60:I = 1 and 2, and the C60:I=12 sample tube contained large I2 crystals after reaction. The sample prepared from C60:I =~3 showed only traces of the strongest C60 reflections and no unreacted iodine. Relative intensities of the reflections from the new compound were essentially the same in all four samples. These observations suggest a single stable phase with greater than 3 iodine equivalents per C60 using the above growth conditions. We also performed thermogravimetric analysis (TGA) on 40 mg of the latter sample and found ca 40% weight loss after heating to 400 deg C, with most of the loss ocurring below 200 deg C. X-ray analysis of the remaining material revealed only the fcc pattern of pure C60. Attributing the loss to iodine and estimating the effect of small masses of unreacted C60 and possible residual iodine yields a composition C60I3.7(+-0.7)
The X-ray data were indexed on a simple hexagonal cell with a = 9.962 Angst.r and c = 9.984 Angstr.. The cell volume per fullerene is 858 Angstr^3 versus 711 Angstr.^3 in fcc C60. Taking 5 Angstr as the fullerene van der Waals radius, this means that 334 Angstr.^3, or 39% of the cell volume, is available for accommodating guest species as opposed to only 26% in the close-packed fcc structure. Iodine atoms (van der Waals radius =~2.2 Angstr.) can fit easily into trigonal prismatic sites at 1/3, 2/3, 1/2 located 7.62 Angstr. away from six C60 centers, or with some crowding into sites with square planar coordination at 1/2, 0, 1/2 located 7.05 Angstr away from four C60 centers. With an atomic volume of 43 Angstr^3, there is ample room for 4 iodine equivalents per cell.
We compared the results of several Rietveld analyses in order to address the following issues: iodine locations and I-I distances for comparison with bond lengths of neutral I2 and typical molecular ions such as I3^-, crystallographic iodine composition for comparison with the TGA and phase mixture results, and possible orientational order of the C60 molecules. Since there are no systematically absent reflections, the symmetry must be P6/mmm or one of its hexagonal or trigonal subgroups. The former, combined with a spherically-averaged C60 form factor , would represent complete orientational disorder, while only the trigonal subgroup P-3 is consistent with the molecular point symmetry if complete orientational order is assumed. We found no significant difference in the quality of the refinements between ordered and disordered models, no doubt due to the large iodine contribution to the total scattering.
The fit to the data was obtained assuming orientational order, trigonal space group P-3. This requires 10 C atoms in the 6g general positions at x, y, z, the coordinates of which were derived assuming 1.39 Angstr and 1.45 Angstr. intramolecular C-C bond lengths. The relative orientation of molecular and crystal axes had little effect on the fit. First we fixed I(1) and I(2) atom positions on the high-symmetry 2d and 3f sites 1/3, 2/3, 1/2 and 1/2, 0, 1/2 respectively, thus constraining the I-I distances to 3.6 Angstr., 5.2 Angstr and 5.9 Angstr which correspond neither to intra- nor intermolecular distances in solid I2 . Not surprisingly, this resulted in a mediocre fit and very large iodine thermal factors, the latter usually indicative of large static displacements. Allowing I(1) and I(2) atoms to relax to positions x, 2x, z with a common thermal factor resulted in the very satisfactory fit (Ri = 8.24%; Rw = 8.32%; thermal factors B(I) = 4.7 Angstr.^2, B(C) = 2.8 Angstr.^2; refined cell constants a = 9.9617(3) Angstr., c = 9.9839(4) Angstr. The refined occupancies are =~2 for each site, corresponding to an ideal formula C60I4, consistent with TGA and phase mixture results.
More significantly, the relaxed I positions yield very reasonable intra- and intermolecular distances. The refined general coordinates of I(1) and I(2) are 0.6423(9), 0.2846(18), +-0.4463(6) and 0.5373(4), 0.0745(8), 0.5 respectively (the z coordinate of I(2) was fixed at its ideal position). These would appear to be randomly displaced from the ideal positions by 0.68 Angstr.\ and 0.64 Angstr respectively. However, locally it is far more plausible that if there is an I(2) atom at 0.5373, 0.0745, 0.5, there will be an I(1) atom on one of the four equivalent sites 0.6423, 0.3577, +-0.4463 or 0.7154, 0.3577, +-0.4463, yielding an I(2) - I(1) distance of 2.53 Angstr., fairly close to the 2.72 Angstr intramolecular distance observed in elemental I2. It is then possible to construct a model in which there are two such I2 molecules per unit cell, with intermolecular distances of 3.78 Angstr. and 5.02 Angstr. but with no long-range order. These intermolecular distances compare favorably with the corresponding values 3.50 Angstr., 3.97 Angstr. and 4.27 Angstr. observed in the complex layer structure of solid I2. The I(1) and I(2) atoms are situated at distances of 7.12 Angstr.and 7.08 Angstr. respectively from the centers of the C60 molecules, and the C-I nearest neighbor separations range from 3.60 Angstr. to 4.0 Angstr.. Associating an occupied I(1)-I(2) pair with I2, the molecules are nearly centered on the trigonal prismatic sites with their axes canted =~11 deg from the midplane.
This model is not only aesthetically pleasing, but all of the distances are completely consistent with a van der Waals packing of I2 which preserves many of the local features of the elemental structure. It is also gratifying to note that the composite structure of C60 and I2 sublattices is essentially that of a stage-1 graphite intercalation compound: an eclipsed sequence (AAA...) of host ``atom'' layers interleaved with guest layers which reside in the van der Waals galleries, as shown schematically in Figure. This suggests that the {111} planes of fcc C60 become the {001} planes in the compound by relative shear motions which transform the layer stacking sequence from ABCABC... to A/A/A/... where ``/'' denotes a guest layer (the eclipsed configuration being stabilized by guest insertion).
In principle, C60I4 should exhibit two kinds of order-disorder transitions. One expects a priori that a C60 orientational ordering transition will occur significantly above the 249 K value observed in pure solid C60 because of the additional steric hindrance from the I2. Additionally, it is possible that at low T the I site occupancy could develop long-range order, most likely accompanied by a dramatic lowering of the symmetry.
The refinement points strongly to molecular (neutral) I2 as the predominant intercalated specie. Saturation doping under similar conditions of a semitransparent C60 film sublimed on mica revealed no gross color changes in transmission or reflection, and no measureable dc conductivity. A search for superconductivity down to 4 K was negative. We conclude, in agreement with Ohno, that solid C60 is oxidized very little if at all by exposure to iodine vapor. On the other hand, we do find strong evidence for the formation of a definite, possibly non-stoichiometric, highly crystalline compound containing substantial iodine. These different findings are no doubt attributable to our reaction conditions in which the compound is allowed to equilibrate with the saturation vapor pressure of the intercalate. Oxidative intercalation of C60 remains an open question; we are currently searching for a spectroscopic signature of small concentrations of molecular ions such as I3^-. For the moment, the new compound C60I4 appears to be more nearly analogous to the uptake of neutral water by clay minerals than to the ionic salts typified by graphite compounds and the alkali-intercalated fullerites. On the other hand, C60I4 does not result from co-crystallization of fullerenes and other molecules (in contrast to the solvated phases reported by Fleming et al. ) and thus should be regarded as a true intercalation compound.
We thank S. Tozer (DuPont) and C. L. Lin (Temple University) for SQUID measurements, and K. S. Liang (Exxon) for the use of the X10B beamline. This work was supported by the NSF Materials Research Laboratory Program under Grant No. DMR88-19885, and by the Department of Energy, Grant Nos. DE-FC02-86ER45254 and DE-FG05-90ER75596. The work at Brookhaven was also supported by DOE, Division of Materials Sciences, DE-AC02-76CH00016.
References
- Fleming, R. M. et al., Nature 352 , 701-703 (1991).
- Zhu, Q. et al. Science 254 , 545-548 (1991).
- Stephens, P.W. et al. Nature 351 , 632-634 (1991).
- Ohno, T. R., Kroll, G. H., Weaver, J. H, Chibante, L. P. F. and Smalley, R. E. Nature 354 , (1991).
- Swinbanks, T. Nature 352 , 749 (1991); ibid. 353 , 377 (1991).
- Lichtenberger, D. L., Nebesny, K. W., Ray, C. W., Huffman, D. R. and Lamb, L. D. Chem. Phys. Letters 176 , 203-208 (1991).
- Dubois, D., Kadish, K. M., Flanagan, S. and Wilson, L. J. J. Am. Chem. Soc. 113 , 7773-7774 (1991).
- Tsukamoto, J., Takahashi, A. and Kawasaki, K. Japanese J. Appl. Physics 29 , 125-130 (1990).
- Fischer, J. E., in Intercalated Layer Materials , edited by F. A. Levy (Reidel, Dordrecht 1979), 481-533.
- Lefrant, S. et al. , Solid State Comm. 29, 191-196 (1979).
- Heiney, P. A. et al. , Phys. Rev. Letters 66, 2911-2914 (1991).
- Neumann, D. A. et al. , Phys. Rev. Letters (in press).
- van Bolhius, F., Koster, P.B. and Migchelsen, T. Acta. Cryst. 23 , 90-91 (1967).
- Fleming, R. M. et al. , Phys. Rev. B44 , 888-891 (1991).